Job Search

Feature Article
Employer RxLogix Japan LLC.

RxLogix Acclaimed by Frost & Sullivan for its Best-in-class 2020 Global Pharmacovigilance Automation Software Product Leadership Award
What We're Proud ofRxLogix Acclaimed by Frost & Sullivan for its Best-in-class 2020 Global Pharmacovigilance Automation Software Product Leadership Award.

RxLogix is a global pharmacovigilance solutions company specializing in innovative software and expert consulting services. Experienced team of business and technology innovators work with Pharmacovigilance and Risk Management Professionals to help increase compliance, productivity and quality for the entire Drug Safety value chain.
Ensuring patient safety while advancing medical and scientific research is vital to life sciences companies. RxLogix highly values defiant bold thinkers entrenched in the world of science, medicine and innovation. From a strategic perspective, RxLogix proactively seeks innovative solutions as preemptive strikes against second-rate thinking.
RxLogix is a one-stop-shop for all PV needs applying most recent technology advances like machine learning, artificial intelligence and best practice methodological approaches.
Recently, after a thorough evaluation of commercially available signaling and analytical software vendors, the FDA selected RxLogix’s PV Surveillance Suite Platform, replacing its legacy FAERS signaling system. FDA has decided to implement RxLogix PV Surveillance Suite (Modules – PV Reports, PV Signal, PV Analytics and PV Datahub) for advanced data analytics, signal detection, evaluation, signal management and benefit-risk assessment.
Safety in Japan
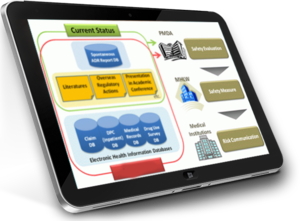
RxLogix was the first company to design the integrated Argus Global-Argus Japan process for large pharma. Since then, RxLogix team has helped several very large to mid-size companies with their Argus implementation projects. RxLogix has the record of the fastest Argus Japan implementation for a top 50 global pharma.
In 2015, RxLogix Corporation and Hitachi Ltd. established a strategic partnership to offer innovative solutions in the Pharmacovigilance and drug safety space for life sciences companies operating in Japan. Hitachi not only helps sell and implement RxLogix' innovative PV software products but also collaborates with RxLogix global and Japan based team for Argus implementation, hosting and managed services projects.
The partnership leverages Hitachi’s tremendous expertise in Japanese PMDA Regulations, significant organizational scale, and local Japanese resources. RxLogix contributes in-depth Argus Safety suite expertise, extensive knowledge of global PV, implementation projects proficiency, data migration capability, and PV presence that includes the United States and Europe. Through the partnership, both companies mobilize their strengths to deliver PV and Drug Safety projects
The FDA Selects RxLogix Corporation Products to Support Safety Surveillance

Princeton, New Jersey, November 19, 2018 – RxLogix Corporation, a global leading provider of Safety and Pharmacovigilance (PV) Software and Services, today announced the signing of an agreement with Booz Allen Hamilton to support the implementation of PV Signal and PV Reports at the US Food and Drug Administration (FDA). The FDA is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices. In order to provide state-of-the-art data analytics and signal management, the Agency will implement the RxLogix PV Signal and PV Reports as part of a larger effort to modernize and enhance the FDA Adverse Event Reporting System (FAERS).